Ultimately, further tests of accuracy can be employed that decide the articles of certain components in the ultimate quantity in the parenteral nutrition admixture. Frequently, pharmacy departments would not have the potential to routinely complete chemical analyses for example analyses of dextrose or electrolyte concentrations. As a result, hospital or institutional laboratories could possibly be called on to carry out these top quality assurance tests. Nonetheless, the solutions in such laboratories in many cases are designed for Organic, not pharmaceutical, devices. Thus, their testing methods needs to be confirmed to fulfill the USP demands stated in the person monograph for that element remaining tested.
If two temperatures are employed for the media-filled units' incubation, the models must be incubated for at least seven days at Every temperature, beginning Together with the reduced temperature. Units are incubated inverted for the first fifty percent in the incubation period of time prior to being returned to an upright posture for the remaining time.
Flooring from the buffer or clear spot are cleaned by mopping once everyday when no aseptic functions are in development. Mopping could possibly be performed by skilled and supervised custodial staff using permitted agents explained during the composed treatments. Only permitted cleaning and sanitizing agents are used with watchful thought of compatibilities, effectiveness, and inappropriate or poisonous residues. Their schedules of use and ways of application are in accord with created methods.
All strategies are executed inside of a method designed to lessen the potential risk of touch contamination. Gloves are sanitized with sufficient frequency with an permitted disinfectant.
I've four pack sizing 20ml,30ml,40ml&100ml what is the frequency for media fill & the best way to demonstrate that previous 6 thirty day period's output was Okay if one pack is not really revalidated within just 6month
Prior to preparing compounded sterile preparations (CSPs) which means all new staff or when you begin a media-fill testing method if you have not presently
All rubber stoppers of vials and bottles and the neck of ampuls are sanitized with IPA before the introduction of a needle or spike for your elimination of products.
Hello Mohammad, it can be strongly check here advised that media fill shall be done inside the apparent transparent bottles. if this provision isn't out there, you'll find various colour medias are in sector which when contaminated could alter the colour and offers a transparent Slash visibility. Delete
These is often carried out upon ask for to ensure the pertinent level of SUSI in critical approach methods.
The articles of our website is usually offered in English and partly in other languages. Opt for your most well-liked language and We'll show you the articles in that language, if out there.
Should the cause is not assignable, then the process should be validated, as It's really a new course of action. Consecutive 3-method simulation test must be read more carried out to demonstrate regularity and dependability around the sterile formulation manufacturing process to generate a suitable product or service.
Scientific studies shall also validate that there is no conversation between product or service and media that may generate cloudiness, precipitate, or other material that would interfere Together with the detection of progress throughout the inspection.
We use cookies on our website to give you the most relevant working experience by remembering your Choices and repeat visits. By clicking “Settle for”, you consent to the use of Each of the cookies. Terms and Conditions and Privacy Policy
Gear It's important that tools, equipment, and gadgets utilized to compound a CSP are persistently capable of running correctly and in acceptable tolerance boundaries. Written treatments outlining needed devices calibration, annual upkeep, checking for suitable function, managed processes for use of the machines and specified time frames for these activities are proven and adopted. Routine upkeep and time intervals are outlined in these composed strategies.
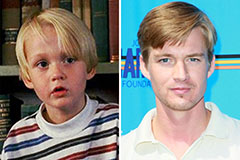 Mason Gamble Then & Now!
Mason Gamble Then & Now! Patrick Renna Then & Now!
Patrick Renna Then & Now! Soleil Moon Frye Then & Now!
Soleil Moon Frye Then & Now! Meadow Walker Then & Now!
Meadow Walker Then & Now! Rossy de Palma Then & Now!
Rossy de Palma Then & Now!